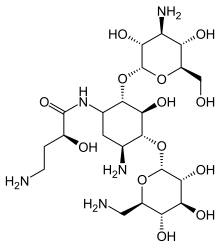
Amikacin
(IUPAC ) ime
(2S )-4-amino-N-[(2S ,3S ,4R ,5S )-5-amino-2-S ,3R ,4S ,5S ,6R )-4-amino-3,5-dihidroksi-R ,3R ,S ,5R ,6R )-6-(aminometil)-3,4,5-trihidroksi-
Klinički podaci
Robne marke
Amikin
AHFS/Drugs.com
Monografija
MedlinePlus
a682661
Identifikatori
CAS broj
37517-28-5
ATC kod
D06 AX12 J01 GB06 S01 AA21
PubChem [ 1] [ 2] 37768
DrugBank
DB00479
ChemSpider [ 3] 34635
UNII
84319SGC3C Y
KEGG [ 4] D02543 Y
ChEBI
CHEBI:2637 Y
ChEMBL [ 5] CHEMBL177 Y
Hemijski podaci
Formula
C 22 H 43 N 5 O 13
Mol. masa
585,603 g/mol
SMILES
eMolekuli PubHem
InChI InChI=1S/C22H43N5O13/c23-2-1-8(29)20(36)27-7-3-6(25)18(39-22-16(34)15(33)13(31)9(4-24)37-22)17(35)19(7)40-21-14(32)11(26)12(30)10(5-28)38-21/h6-19,21-22,28-35H,1-5,23-26H2,(H,27,36)/t6-,7+,8-,9+,10+,11-,12+,13+,14+,15-,16+,17-,18+,19-,21+,22+/m0/s1 Y Y
Farmakokinetički podaci
Vezivanje za proteine plazme
0-11%
Poluvreme eliminacije
2-3 sata
Izlučivanje
Renalno
Farmakoinformacioni podaci
Trudnoća
D(AU ) C(US )
Pravni status
POM (UK ) ℞ -only (SAD )
Način primene
Intramuskularno , intravenozno
Amikacin je aminoglikozidni antibiotik koji se koristi za tretiranje različitih tipova bakterijskih infekcija . Amikacin deluje putem vezivanja za bakterijsku 30S ribozomalnu podjedinicu, čime uzrokuje pogrešno očitavanje iRNK te time onemogućava bakterijsku sintezu proteina koji su vitalni za njen rast.
Medicinske upotrebe
Amikacin se najčešće koristi za tretiranje ozbiljnih, u bolnici stečenih infekcija uzrokovanih gramnegativnim bakterijama otpornim na višestruke lekove kao što su Pseudomonas aeruginosa Acinetobacter Enterobacter Serratia marcescens Providencia stuartii
Amikacin se može kombinovati sa beta-laktamskim antibioticima za terapiju ljudi sa neutropenijom i groznicom .
Lipozomalni amikacin za inhaliranje je trenutno u završnom stadijumu kliničkih ispitivanja za tretman respiratornih bolesti, kao što je cistična fibroza [ 6] Pseudomonas aeruginosa [ 7] [ 8] bronhiektazije .[ 9] [ 10]
Reference
↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.” . Drug Discov Today 15 (23-24): 1052-7. DOI :10.1016/j.drudis.2010.10.003 . PMID 20970519 . edit ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4 : 217-241. DOI :10.1016/S1574-1400(08)00012-1 . ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining” . J Cheminform 2 (1): 3. DOI :10.1186/1758-2946-2-3 . PMID 20331846 . edit ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG” . Yeast 17 (1): 48–55. DOI :10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H . ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI :10.1093/nar/gkr777 . PMID 21948594 . edit ↑ "Randomized, open-label, active-controlled, multicenter study to assess the efficacy, safety and tolerability of Arikace™ in Cystic Fibrosis patients with chronic infection due to Pseudomonas aeruginosa" is a European Phase III clinical trial, being conducted across multiple sites in the EU, starting at the Royal Brompton Hospital, Department of Respiratory Medicine, in London↑ Study to Evaluate Arikace™ in CF Patients With Chronic Pseudomonas Aeruginosa Infections ↑ "A Randomized, Double-Blind, Placebo-Controlled Study of Liposomal Amikacin for Inhalation (Arikace™) in Patients With Recalcitrant Nontuberculous Mycobacterial Lung Disease" is a Phase II clinical trial in collaboration with the US National Institute of Allergy and Infectious Diseases↑ "A Placebo Controlled, Randomized, Parallel Cohort, Safety And Tolerability Study Of 2 Dose Levels Of Liposomal Amikacin For Inhalation (Arikace™) In Patients With Bronchiectasis Complicated By Chronic Infection Due To Pseudomonas Aeruginosa" Phase II (completed).↑ "A Study to Determine the Safety and Tolerability of Arikace™ Versus Placebo in Patients Who Have Bronchiectasis" Arhivirano 2015-04-29 na Wayback Machine-u is a Phase II clinical trial (as [4]) completed in the UK.
Literatura
Edson RS, Terrell CL. The aminoglycosides. Mayo Clin Proc. 1999 May;74(5):519-28. Review. PMID 10319086
Opšte: Geometrija Monosaharidi
Ketoheksoze (
Psikoza ,
Fruktoza ,
Sorboza ,
Tagatoza )
Aldoheksoze (Aloza , Altroza , Glukoza , Manoza , Guloza , Idoza , Galaktoza , Taloza )
Dezoksi šećeri (
Fukoza ,
Fukuloza ,
Ramnoza )
>6
Višestruki
Information related to Amikacin